Background. Maintaining remission in AML after intensive chemotherapy continues to be a challenge, specifically in patients with measurable residual disease (MRD+). Immunotherapy could be an effective way to induce durable disease control for patients in remission. This phase 2 study used an allogenic leukemia-derived dendritic cell vaccine to induce an effective anti-leukemic response to control or eradicated MRD, leading to durable disease control. (ADVANCE-II, Clintrials.gov: NCT03697707).
Methods. A total of 20 evaluable AML patients, in complete remission (CR1/CRi), MRD + and ineligible for allo-HSCT at inclusion, were scheduled to receive 4 biweekly doses of vididencel, followed by 2 booster doses at week 14 and 18. Patients aged 34-79 (median 60 years) had an ELN2017 risk score assessed of 13 favorable, 6 intermediate and 1 adverse, with mutations in NPM1 (n=10), CBFB-MYH11 (n=4), IDH2 (n=2), RUNX1-RUNX1T1 (n=2), TP53 (n=1) and CEBPA (n=1). Patients were vaccinated after a median of 8.9 months (range 3 to 60.8 Mo) after reaching CR1/CRi.
MRD was assessed at baseline, week 14, 20 and 32 by flow cytometry and/or molecular analyses on bone marrow samples. Immune responses were evaluated on peripheral blood samples taken before and during treatment by a) IFNγ ELISPOT using three antigens (WT1, PRAME and RHAMM), known to be expressed by vididencel, by b) flow cytometry, using a single 40-marker panel, and c) skin biopsies taken 2 days after the first, fourth and sixth dose to assess immune responses in the skin. Evaluation of relapse-free and overall survival was done at data cut-off (3 rd July 2023).
Results. All 20 patients have received 4 initial vaccinations, 17 patients received all booster vaccinations and 1 patient received only one booster vaccination. No serious adverse events (AE) or severe AE (grade 3 or higher) related to the treatment have been reported. Related AEs are mainly injection site reactions, such as redness, warmth and swelling, occurring within 48 hours after intradermal administration.
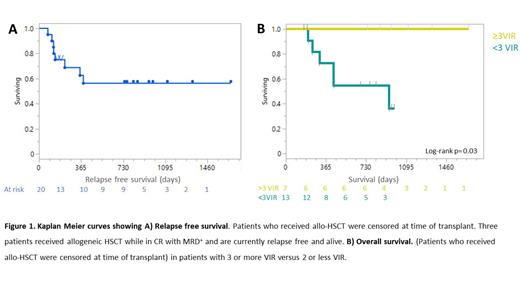
To date, after a median follow-up of 24.8 Mo (range 6.6- 54.8) (N=20) 14 patients are still alive with 12 being relapse-free for 24.4 to 54.7 months. The median RFS and OS (not shown) have not yet been reached (Figure 1A). Estimated RFS and OS at 12 months is 73% (range 51-86%) and 87% (range 67-95%), respectively, whereas the 24 months RFS is 59% (range 35-77%) and OS is 73% (range 50-87%).
Induction of at least one vaccine induced response (VIR), as measured by IFNγ ELISPOT, was observed in 17 out of 20 patients, with a sustained vaccine induced response (sVIR) in 9 patients. There is a clear correlation (LogRank p=0.03) between number of VIRs and overall survival (Figure 1B), with best survival in patients with at least 3 VIRs. Of these patients, 4 out of 7 had shown MRD conversion (MRD -). Analysis of the immune cell composition at baseline and after vaccination revealed that higher baseline levels of B-cells (p <0.05) , cDC1, cDC2 were observed in patients remaining in CR. Vaccination also improved immune cell frequencies of dendritic cells with highest levels correlating with longer RFS (p<0.05) and OS (p<0.05). Immune histochemistry on skin biopsies confirmed the large influx of CD4+ and CD8+ T-cells as well as antigen presenting cells, in all patients explaining the clinical observation of swelling, warmth and redness in almost all patients.
Conclusion/discussion. Vaccination with vididencel resulted in durable disease control, reflected by 2-year RFS and OS of 59% and 73%, respectively and the emerging plateau in the corresponding Kaplan Meier curves. Immune response assays indicate the induction of a strong immune response, with induction of specific T-cell responses towards tumor associated antigens, increased frequencies of B-cells and dendritic cells, all correlating with longer RFS and OS. The locally skin induced immune response leading to influx of a plethora of immune cells, confirmed the mode of action of immune priming through intradermal administration of the cancer vaccine. These results warrant further studies combining vididencel with SOC such as hypomethylating agents in AML maintenance.
Disclosures
van de Loosdrecht:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding. Platzbecker:Geron: Consultancy, Research Funding; AbbVie: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Janssen Biotech: Consultancy, Research Funding; Merck: Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Curis: Consultancy, Research Funding; Fibrogen: Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Amgen: Consultancy, Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Holderried:Neovii: Other: Travel, accomodation, expenses; Astellas: Other: Travel, accomodation, expenses; Jazz: Consultancy, Honoraria, Other: Travel, accomodation, expenses; AbbVie: Other; Janssen: Other: Travel, accomodation, expenses; Pfizer: Consultancy; Sanofi: Consultancy, Other: Travel, accomodation, expenses; Novartis: Consultancy; Amgen: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Other: Travel, accomodation, expenses; Bristol-Myers-Squibb: Consultancy, Honoraria, Other: Travel, accomodation, expenses; Kite/Gilead: Consultancy, Other: Travel, accomodation, expenses. Giagounidis:Novartis: Consultancy; Keros Pharmaceuticals: Consultancy; Amgen: Consultancy; Curis: Consultancy; BMS: Consultancy. Van Zeeburg:Mendus AB: Current Employment. Rovers:Mendus AB: Current Employment, Current equity holder in publicly-traded company. Gjertsen:BerGenBio: Consultancy; GreinDX: Consultancy; Immedica: Consultancy; InCyte: Consultancy; Mendus AB: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Otsuka: Consultancy; Pfizer: Consultancy, Research Funding; Sanofi: Consultancy; in Alden Cancer Therapy AS: Current holder of stock options in a privately-held company; KinN Therapeutics AS: Current holder of stock options in a privately-held company; Coegin: Consultancy.